Solvents don’t just dissolve other chemicals (called solutes) and then sit around with their hands in their pockets. Instead, they get involved in all sorts of different ways when dissolved molecules toss electrons around, i.e., they facilitate charge transfer events. In research, the hard part is fi guring out exactly how and when solvent molecules get involved when an electron hops from one solute molecule to another. For example, in liquids (which do most of the dissolving), solvent molecules move constantly, making it very challenging to see what they’re doing when charge transfer events occur.
The Lineberger group recently met this challenge using a simple prototype gas-phase system. In this system, a single solvent molecule of carbon dioxide (CO2) interacts with an IBr- molecule as it is broken apart by a laser pulse. A second, or probe, laser pulse is used to watch what happens as the IBr- molecule falls apart. The group discovered the ways in which the CO2 molecule affected the dissociation process. The experiments were performed by research associate Lenny Sheps and graduate student Elisa Miller under the guidance of Fellow Carl Lineberger. The experimentalists collaborated with theorists Robert Parson, former graduate student Matt Thompson, Anne McCoy (the Ohio State University) and McCoy’s student Samantha Horvath to unravel the role of CO2 in the dissociation process.
When there was no solvent (CO2) present, the laser pulse moved the IBr- into a specifi c electronic state that then fell apart, producing only I- and Br atoms. However, with a single CO2 molecule present, things got very interesting. About a third of the time, the breakup still produced only I- and Br atoms. The CO2 molecule basically flew off into space. Then, a little less than two-thirds of the time, the breakup produced an I-(CO2) complex and a Br atom. In this case, the CO2 molecule kind of snuggled up to the I- atom during the dissociation and just kept vibrating against it.
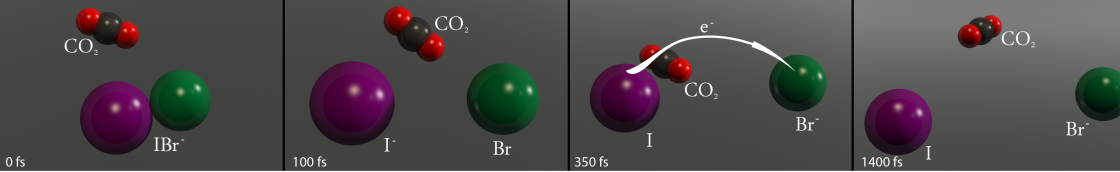
The most interesting chain of events occurred only about 3% of the time. In this case, right after the IBr- was hit by the fi rst laser pulse, the I-, Br, and CO2 began to separate, much like in the pathway that produced just a Br atom and an I-(CO2) complex. However, in this case, the I-(CO2) complex was hot (rapidly vibrating). After about 350 fs, it slammed against the Br atom, and the CO2 formed a bridge between the I- and Br atoms, which had been in the process of separating.
When the two atoms were about 7 Å apart (which is really far in the world of atoms and molecules), the extra electron on the iodine atom hopped right across the CO2 bridge and into the Br atom, producing I and Br- atoms. But, the CO2 solvent molecule did more than just provide a physical bridge between the two atoms for the hopping electron. It also absorbed the energy released when the electron combined with the Br atom. After the charge transfer was complete, the CO2 molecule was left vibrating like crazy, which pushed it away from the two atoms.
This seminal experiment not only showed that a single solvent molecule can facilitate charge transfer, but also exactly how it can perform this feat. The Lineberger group is currently investigating charge transfer mediated by two solvent molecules. - Julie Phillips


 The Physics Frontiers Centers (PFC) program supports university-based centers and institutes where the collective efforts of a larger group of individuals can enable transformational advances in the most promising research areas. The program is designed to foster major breakthroughs at the intellectual frontiers of physics by providing needed resources such as combinations of talents, skills, disciplines, and/or specialized infrastructure, not usually available to individual investigators or small groups, in an environment in which the collective efforts of the larger group can be shown to be seminal to promoting significant progress in the science and the education of students. PFCs also include creative, substantive activities aimed at enhancing education, broadening participation of traditionally underrepresented groups, and outreach to the scientific community and general public.
The Physics Frontiers Centers (PFC) program supports university-based centers and institutes where the collective efforts of a larger group of individuals can enable transformational advances in the most promising research areas. The program is designed to foster major breakthroughs at the intellectual frontiers of physics by providing needed resources such as combinations of talents, skills, disciplines, and/or specialized infrastructure, not usually available to individual investigators or small groups, in an environment in which the collective efforts of the larger group can be shown to be seminal to promoting significant progress in the science and the education of students. PFCs also include creative, substantive activities aimed at enhancing education, broadening participation of traditionally underrepresented groups, and outreach to the scientific community and general public.